By V. Virvilis and E. Lekka
The latest release of Biovista Vizit (September 2025) features the integration of the ClinicalTrials.gov database.
This is the fourth public corpus integration of Biovista Vizit alongside MEDLINE following HPO, AOP and PDB.
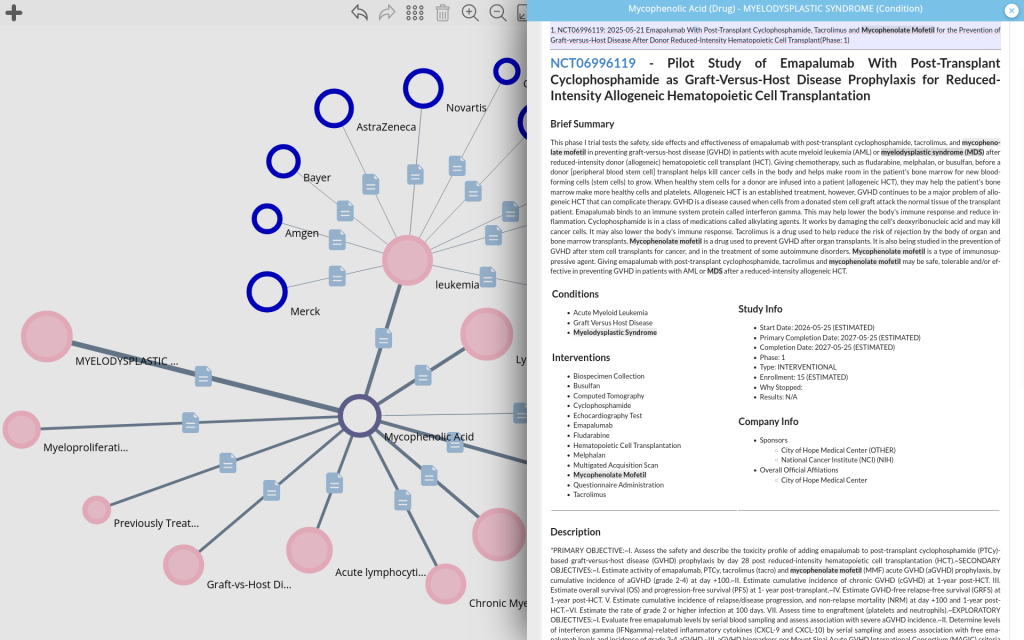
This integration makes a significant effort to address the irregular and non-standardized manner in which data are entered in the ClinicalTrials.gov database [1, 2, 3] delivering high-quality synonymization and consolidation of non-normalized terms.
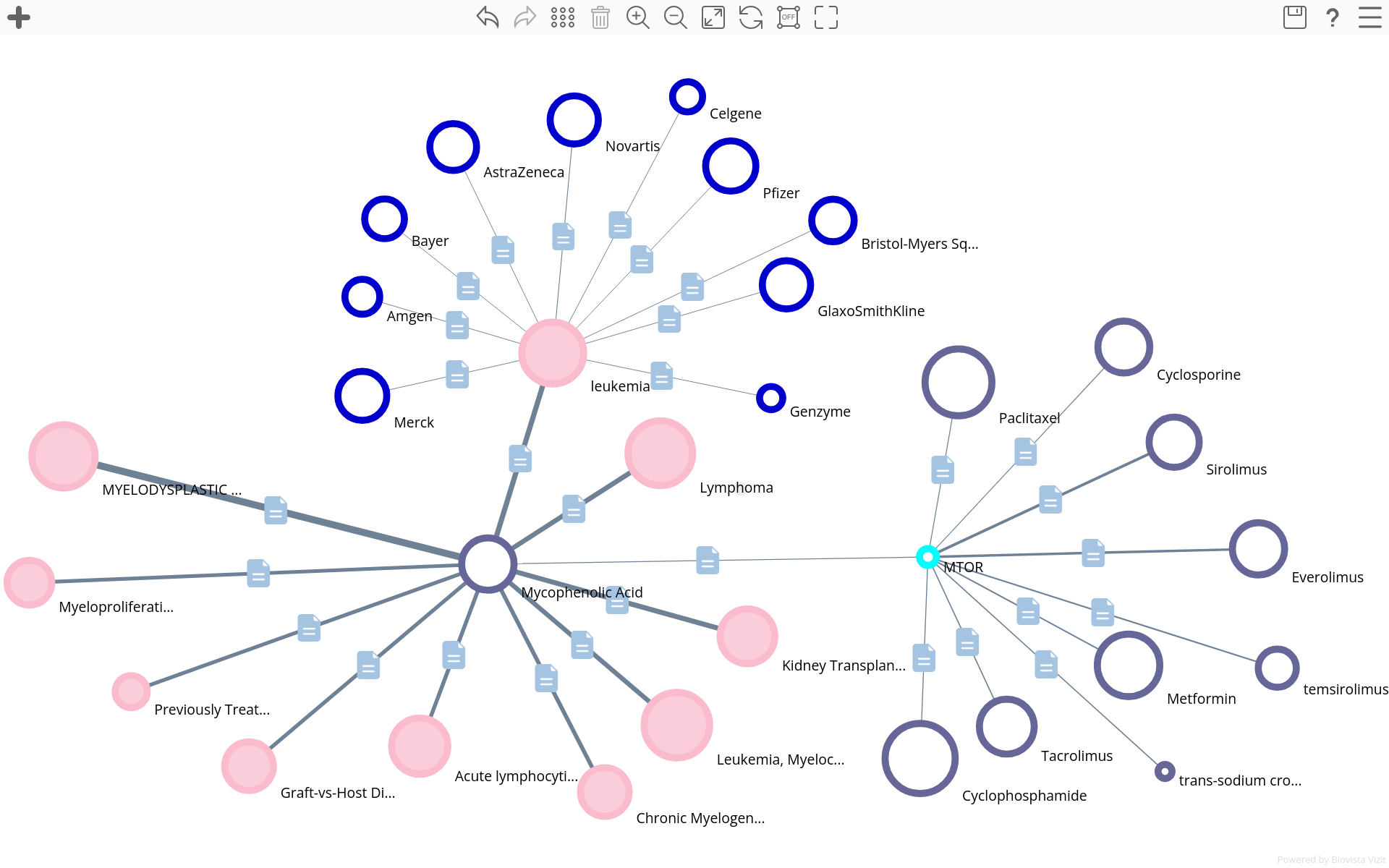
Biovista Vizit introduces two new Entity Types:
- Conditions
- Interventions
These additions enhance corpus mapping while preserving the original terminology present in the source data. Both new types are cross-linked to the well-established existing Entity Types such as:
- Adverse Events
- Genes
- Pathways
- Companies
- etc.
As always each link is backed by each corresponding supporting evidence.
This integration is offered by request. Please use our contact form to arrange a demo and explore its features.
Reference
- Hoyt CT, Hoyt AL, Gyori BM. Prediction and curation of missing biomedical identifier mappings with Biomappings. Bioinformatics. 2023 Apr 3;39(4):btad130. https://doi: 10.1093/bioinformatics/btad130
- Liu, H., Carini, S., Chen, Z., Phillips Hey, S., Sim, I., & Weng, C. (2022). Ontology-based categorization of clinical studies by their conditions. Journal of Biomedical Informatics, 135, 104235. https://doi.org/10.1016/j.jbi.2022.104235
- Miron, L., Gonçalves, R.S. & Musen, M.A. Obstacles to the reuse of study metadata in ClinicalTrials.gov. Sci Data 7, 443 (2020). https://doi.org/10.1038/s41597-020-00780-z